Nutrition as it relates to your Enzymes
The Wonders of Digestive Enzymes Metabolic Tool for You!
Nutrition as it relates to your Enzymes are proteins made by cells in our bodies and by all living organisms.
They are specialized proteins that do work, such as synthesizing chemicals, rearranging molecules, adding elements to compounds, and breaking down compounds.
Enzymes are protein catalysts. They cause biological reactions to occur under conditions that sustain life. Many types of enzymes exist, and each type does one specific function.
For an enzyme to work, the material it works upon must be present. If no such material is available to the enzyme, the enzyme performs no function.
There are also metabolic enzymes in our body, but this discussion is limited to the digestive enzymes that help break down food.
Nutrition as it relates to your enzymes, and How do They Work?
In general, enzymes work as catalysts of biochemical reactions. A catalyst increases or accelerates the rate of a reaction.
The thousands of chemical reactions that occur in our body every second could not happen without enzymes to speed up these reactions.
For example, a protein can be broken down into amino acids in the lab without the use of an enzyme, but to do so requires extreme temperatures, high pressure, or very strong acids; conditions not compatible with life.
Even with these conditions, it often requires hours to complete the reaction in the lab. Enzymes, in this case a mixture of proteases, can complete this reaction within minutes in water at normal temperatures.
Another unique aspect of enzymes is that they facilitate the reaction without being destroyed or changed in the process. Because of this, one enzyme molecule could theoretically change an infinite amount of substrate if given an infinite amount of time.
Increasing the amount of enzyme decreases the time required for completing the process. If you double the number of enzyme molecules, you decrease the time for the reaction by half.
The Central Role of Enzymes as Biological Catalysts
A fundamental task of proteins is to act as enzymes—catalysts that increase the rate of virtually all the chemical reactions within cells. Although RNAs are capable of catalyzing some reactions, most biological reactions are catalyzed by proteins. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of temperature and pressure that are compatible with life.
Enzymes accelerate the rates of such reactions by well over a million-fold, so reactions that would take years in the absence of catalysis can occur in fractions of seconds if catalyzed by the appropriate enzyme. Cells contain thousands of different enzymes, and their activities determine which of the many possible chemical reactions actually take place within the cell.
The Catalytic Activity of Enzymes
Like all other catalysts, enzymes are characterized by two fundamental properties. First, they increase the rate of chemical reactions without themselves being consumed or permanently altered by the reaction. Second, they increase reaction rates without altering the chemical equilibrium between reactants and products.
These principles of enzymatic catalysis are illustrated in the following example, in which a molecule acted upon by an enzyme (referred to as a substrate [S]) is converted to a product (P) as the result of the reaction. In the absence of the enzyme, the reaction can be written as follows:
The chemical equilibrium between S and P is determined by the laws of thermodynamics (as discussed further in the next section of this chapter) and is represented by the ratio of the forward and reverse reaction rates (S→P and P→S, respectively).
In the presence of the appropriate enzyme, the conversion of S to P is accelerated, but the equilibrium between S and P is unaltered. Therefore, the enzyme must accelerate both the forward and reverse reactions equally. The reaction can be written as follows:
Note that the enzyme (E) is not altered by the reaction, so the chemical equilibrium remains unchanged, determined solely by the thermodynamic properties of S and P.
The effect of the enzyme on such a reaction is best illustrated by the energy changes that must occur during the conversion of S to P
(Figure 2.22).
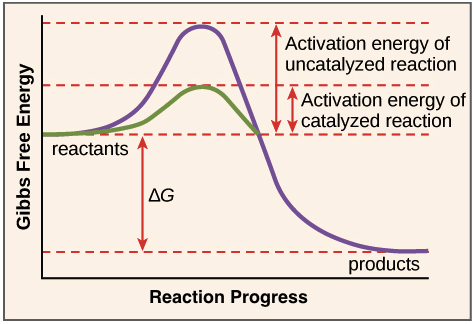
The equilibrium of the reaction is determined by the final energy states of S and P, which are unaffected by enzymatic catalysis. For the reaction to proceed, however, the substrate must first be converted to a higher energy state, called the transition state.
The energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction.
Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction.
The increased rate is the same in both the forward and reverse directions, since both must pass through the same transition state.
Energy diagrams for catalyzed and uncatalyzed reactions
The reaction illustrated is the simple conversion of a substrate S to a product P. Because the final energy state of P is lower than that of S, the reaction proceeds from left to right.
For the reaction to occur, however, S must first pass through a higher energy transition state. The energy required to reach this transition state (the activation energy) represents a barrier to the progress of the reaction and thereby determines the rate at which the reaction proceeds. In the presence of a catalyst (e.g., an enzyme), the activation energy is lowered and the reaction proceeds at an accelerated rate.
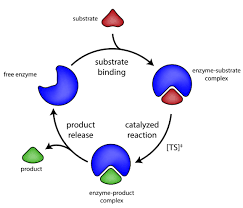
The catalytic activity of enzymes involves the binding of their substrates to form an enzyme-substrate complex (ES). The substrate binds to a specific region of the enzyme, called the active site. While bound to the active site, the substrate is converted into the product of the reaction, which is then released from the enzyme. The enzyme-catalyzed reaction can thus be written as follows:
Note that E appears unaltered on both sides of the equation, so the equilibrium is unaffected. However, the enzyme provides a surface upon which the reactions converting S to P can occur more readily. This is a result of interactions between the enzyme and substrate that lower the energy of activation and favor formation of the transition state.
Mechanisms of Enzymatic Catalysis
The binding of a substrate to the active site of an enzyme is a very specific interaction. Active sites are clefts or grooves on the surface of an enzyme, usually composed of amino acids from different parts of the polypeptide chain that are brought together in the tertiary structure of the folded protein.
Substrates initially bind to the active site by noncovalent interactions, including hydrogen bonds, ionic bonds, and hydrophobic interactions. Once a substrate is bound to the active site of an enzyme, multiple mechanisms can accelerate its conversion to the product of the reaction.
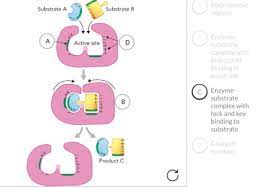
Although the simple example discussed in the previous section involved only a single substrate molecule, most biochemical reactions involve interactions between two or more different substrates.
For example, the formation of a peptide bond involves the joining of two amino acids. For such reactions, the binding of two or more substrates to the active site in the proper position and orientation accelerates the reaction (Figure 2.23).
The enzyme provides a template upon which the reactants are brought together and properly oriented to favor the formation of the transition state in which they interact.
Figure 2.23 Enzymatic catalysis of a reaction between two substrates.
The enzyme provides a template upon which the two substrates are brought together in the proper position and orientation to react with each other.
Enzymes accelerate reactions also by altering the conformation of their substrates to approach that of the transition state. The simplest model of enzyme-substrate interaction is the lock-and-key model, in which the substrate fits precisely into the active site (Figure 2.24).
In many cases, however, the configurations of both the enzyme and substrate are modified by substrate binding—a process called induced fit. In such cases the conformation of the substrate is altered so that it more closely resembles that of the transition state.
The stress produced by such distortion of the substrate can further facilitate its conversion to the transition state by weakening critical bonds.
Moreover, the transition state is stabilized by its tight binding to the enzyme, thereby lowering the required energy of activation.
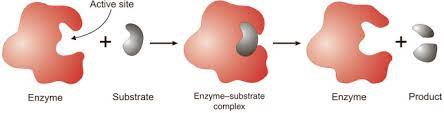
Figure 2.24 Models of enzyme-substrate interaction
(A) In the lock-and-key model, the substrate fits precisely into the active site of the enzyme.
(B) In the induced-fit model, substrate binding distorts the conformations of both substrate and enzyme.
This distortion brings the substrate closer to the conformation of the transition state, thereby accelerating the reaction.
In addition to bringing multiple substrates together and distorting the conformation of substrates to approach the transition state, many enzymes participate directly in the catalytic process.
In such cases, specific amino acid side chains in the active site may react with the substrate and form bonds with reaction intermediates.
The acidic and basic amino acids are often involved in these catalytic mechanisms, as illustrated in the following discussion of chymotrypsin as an example of enzymatic catalysis.
Chymotrypsin is a member of a family of enzymes (serine proteases) that digest proteins by catalyzing the hydrolysis of peptide bonds. The reaction can be written as follows:
The different members of the serine protease family (including chymotrypsin, trypsin, elastase, and thrombin) have distinct substrate specificities; they preferentially cleave peptide bonds adjacent to different amino acids.
For example, whereas chymotrypsin digests bonds adjacent to hydrophobic amino acids, such as tryptophan and phenylalanine, trypsin digests bonds next to basic amino acids, such as lysine and arginine.
All the serine proteases, however, are similar in structure and use the same mechanism of catalysis.
The active sites of these enzymes contain three critical amino acids—serine, histidine, and aspartate—that drive hydrolysis of the peptide bond.
Indeed, these enzymes are called serine proteases because of the central role of the serine residue.
Substrates bind to the serine proteases by insertion of the amino acid adjacent to the cleavage site into a pocket at the active site of the enzyme (Figure 2.25).
The nature of this pocket determines the substrate specificity of the different members of the serine protease family.
For example, the binding pocket of chymotrypsin contains hydrophobic amino acids that interact with the hydrophobic side chains of its preferred substrates.
In contrast, the binding pocket of trypsin contains a negatively charged acidic amino acid (aspartate), which is able to form an ionic bond with the lysine or arginine residues of its substrates.
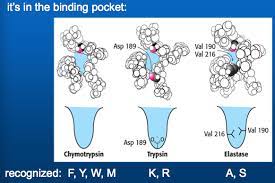
Figure 2.25 Substrate binding by serine proteases
The amino acid adjacent to the peptide bond to be cleaved is inserted into a pocket at the active site of the enzyme. In chymotrypsin, the pocket binds hydrophobic amino acids; the binding pocket of trypsin contains a negatively charged aspartate residue that binds basic amino acids via an ionic interaction.
Substrate binding positions the peptide bond to be cleaved adjacent to the active site serine (Figure 2.26). The proton of this serine is then transferred to the active site histidine. The conformation of the active site favors this proton transfer because the histidine interacts with the negatively charged aspartate residue.
The serine reacts with the substrate, forming a tetrahedral transition state. The peptide bond is then cleaved, and the C-terminal portion of the substrate is released from the enzyme. However, the N-terminal peptide remains bound to serine. This situation is resolved when a water molecule (the second substrate) enters the active site and reverses the preceding reactions.
The proton of the water molecule is transferred to histidine, and its hydroxyl group is transferred to the peptide, forming a second tetrahedral transition state. The proton is then transferred from histidine back to serine, and the peptide is released from the enzyme, completing the reaction.
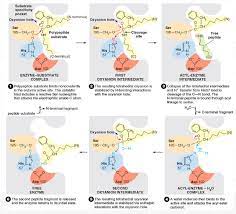
Catalytic mechanism of chymotrypsin. Three amino acids at the active site (Ser-195, His-57, and Asp-102) play critical roles in catalysis.
This example illustrates several features of enzymatic catalysis; the specificity of enzyme-substrate interactions, the positioning of different substrate molecules in the active site, and the involvement of active-site residues in the formation and stabilization of the transition state.
Although the thousands of enzymes in cells catalyze many different types of chemical reactions, the same basic principles apply to their operation.
Coenzymes
In addition to binding their substrates, the active sites of many enzymes bind other small molecules that participate in catalysis. Prosthetic groups are small molecules bound to proteins in which they play critical functional roles. For example, the oxygen carried by myoglobin and hemoglobin is bound to heme, a prosthetic group of these proteins.
In many cases metal ions (such as zinc or iron) are bound to enzymes and play central roles in the catalytic process. In addition, various low-molecular-weight organic molecules participate in specific types of enzymatic reactions. These molecules are called coenzymes because they work together with enzymes to enhance reaction rates.
In contrast to substrates, coenzymes are not irreversibly altered by the reactions in which they are involved. Rather, they are recycled and can participate in multiple enzymatic reactions.
Coenzymes serve as carriers of several types of chemical groups. A prominent example of a coenzyme is nicotinamide adenine dinucleotide (NAD+), which functions as a carrier of electrons in oxidation-reduction reactions (Figure 2.27).
NAD+ can accept a hydrogen ion (H+) and two electrons (e-) from one substrate, forming NADH. NADH can then donate these electrons to a second substrate, re-forming NAD+. Thus, NAD+ transfers electrons from the first substrate (which becomes oxidized) to the second (which becomes reduced).
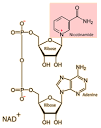
(A) Nicotinamide adenine dinucleotide (NAD+) acts as a carrier of electrons in oxidation-reduction reactions by accepting electrons (e-) to form NADH. (B) For example, NAD+ can accept electrons from one substrate (S1), yielding oxidized S1 plus NADH.
The NADH formed in this reaction can then transfer its electrons to a second substrate (S2), yielding reduced S2 and regenerating NAD+. The net effect is the transfer of electrons (carried by NADH) from S1 (which becomes oxidized) to S2 (which becomes reduced).
Several other coenzymes also act as electron carriers, and still others are involved in the transfer of a variety of additional chemical groups (e.g., carboxyl groups and acyl groups; Table 2.1). The same coenzymes function together with a variety of different enzymes to catalyze the transfer of specific chemical groups between a wide range of substrates.
Many coenzymes are closely related to vitamins, which contribute part or all of the structure of the coenzyme. Vitamins are not required by bacteria such as E. coli but are necessary components of the diets of human and other higher animals, which have lost the ability to synthesize these compounds.
Regulation of Enzyme Activity
An important feature of most enzymes is that their activities are not constant but instead can be modulated. That is, the activities of enzymes can be regulated so that they function appropriately to meet the varied physiological needs that may arise during the life of the cell.
One common type of enzyme regulation is feedback inhibition, in which the product of a metabolic pathway inhibits the activity of an enzyme involved in its synthesis. For example, the amino acid isoleucine is synthesized by a series of reactions starting from the amino acid threonine (Figure 2.28).
The first step in the pathway is catalyzed by the enzyme threonine deaminase, which is inhibited by isoleucine, the end product of the pathway. Thus, an adequate amount of isoleucine in the cell inhibits threonine deaminase, blocking further synthesis of isoleucine.
If the concentration of isoleucine decreases, feedback inhibition is relieved, threonine deaminase is no longer inhibited, and additional isoleucine is synthesized. By so regulating the activity of threonine deaminase, the cell synthesizes the necessary amount of isoleucine but avoids wasting energy on the synthesis of more isoleucine than is needed.
Feedback inhibition. The first step in the conversion of threonine to iso-leucine is catalyzed by the enzyme threonine deaminase. The activity of this enzyme is inhibited by isoleucine, the end product of the pathway.